Purpose
To learn how to use the source document for the TMS session as part of VERIFY. There is no quiz or certification for this module.
Overview
The source document for the TMS test is provided as a PDF.
You should print the source document out and fill it in during the TMS test. The data on the source document should be used to complete the TMS CRF on WebDCU once the TMS session is completed. Please write names exactly as they are listed on the DOA.
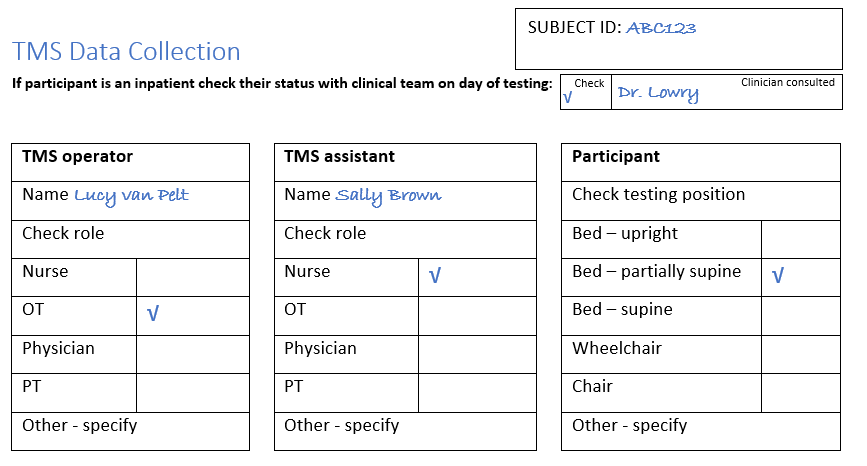
The first section of the source document collects data about the TMS session. An example is provided below.
This hypothetical session takes place at 1.30pm on the 28th of October.
The participant’s right upper limb is paretic and was tested.
Lucy van Pelt is an OT and she is the TMS Operator for the session, and the TMS Assistant was Sally Brown who is a nurse.
The participant was partially supine in their bed at the time of testing.
The patient had already provided written informed consent, and their TMS Safety Screening Checklist was completed with no contraindications to TMS present. On the day of TMS testing the patient’s physician Dr. Lowry confirmed testing was still appropriate.
Lucy and Sally set up EMG recording from the patient’s right ECR and FDI muscles and ensured the EMG signals were free from biological and non-biological noise.
Lucy explained to the patient what they could expect, and positioned the TMS coil over the patient’s left primary motor cortex. The center of the coil was initially positioned about 2 inches lateral to the vertex, on the inter-aural line, with the handle pointing backwards at an angle around 45 degrees.
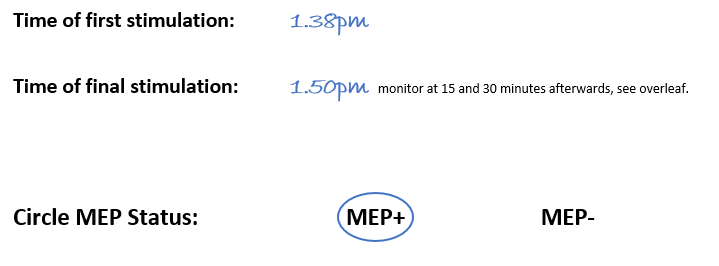
Lucy started stimulation at 1.38pm and this time was noted on the source document. Lucy started with an intensity of 30% MSO and systematically moved the coil in half-inch steps, but no MEPs were observed. So she increased the intensity to 40%, and then 50%, however no MEPs were observed at either intensity.
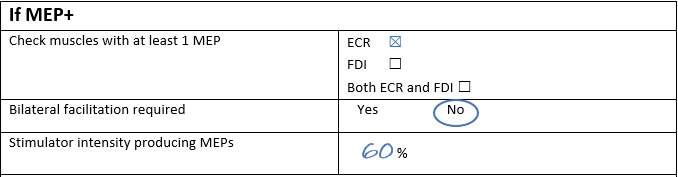
Lucy then increased the intensity to 60% and noticed a small MEP in the ECR EMG trace. Lucy kept the TMS coil in the same position and delivered 9 more stimulations at 60% intensity, and 6 of these resulted in MEPs in the ECR EMG trace. Sally recorded the patient’s MEP status as MEP+ on the source document.
Throughout the TMS session Lucy noted no MEPs were elicited in the FDI and so she checked “ECR” for the question “Check muscles with at least 1 MEP,” and this can be seen below. If Lucy had elicited any FDI MEP at any intensity during the TMS session then she would’ve marked “both ECR and FDI.”
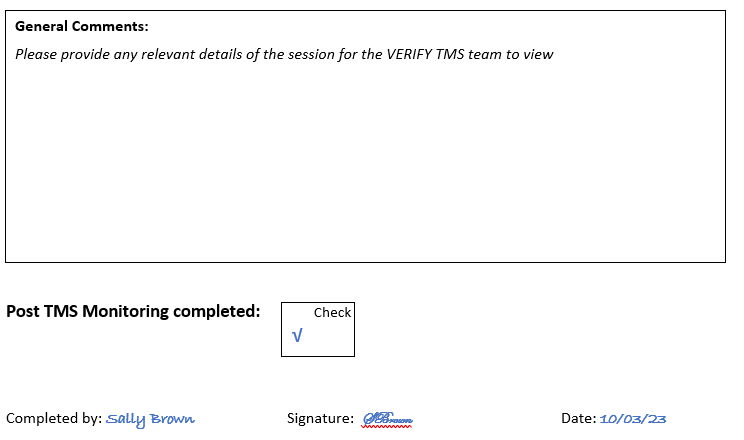
Sally noted on the source document that the final stimulation was delivered at 1.50pm. The patient has to be clinically monitored at 15 minutes and 30 minutes after TMS so Sally knew that the patient needed to be clinically monitored at 2.05pm and 2.20pm.
Once the clinical monitoring was complete Sally noted this on the source document, and named and signed it as the person who completed the source document.
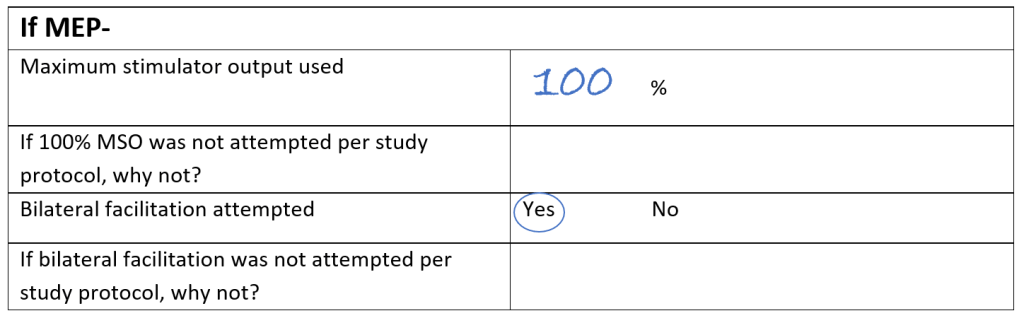
Here’s another example, but this time the patient was MEP-. You can see that the maximum stimulator output used was 100% MSO, and that the patient was asked to hug a pillow to facilitate the corticomotor system. It was documented in the comments section that all 10 stimulations at 100% intensity during bilateral facilitation were delivered in different scalp locations to demonstrate they performed systemic coil positioning. No MEPs were observed at 100% intensity during bilateral facilitation with systemic coil positioning so this patient was recorded as MEP-.
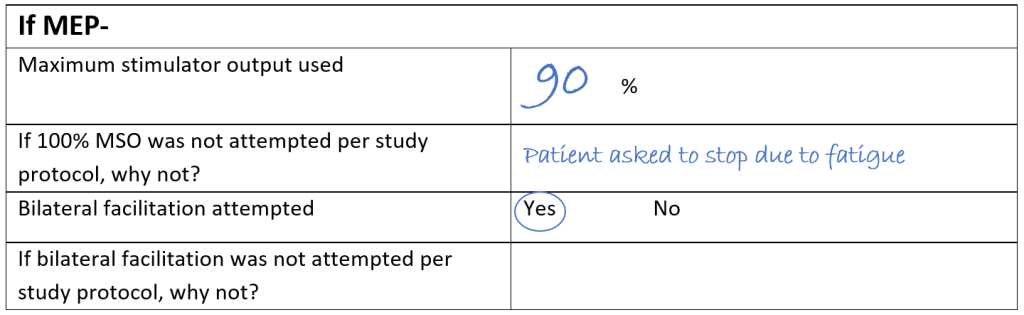
And finally, here’s an example of a MEP- patient where 90% MSO was the maximum stimulator used. Sally noted that 100% MSO wasn’t used because the patient asked to stop the session due to fatigue but facilitation was still attempted, and this means the MEP- status determination is not usable. In order to try obtain a usable MEP status determination a TMS session for this patient was rescheduled for the following day which was still between 72-168 hours post-stroke.
Monitoring the participant after TMS
It is important to monitor VERIFY patients for any adverse effects of TMS. Healthy volunteers do not require clinical monitoring after TMS.
What are the risks?
TMS is considered safe and very low risk for participants who pass the TMS Safety Screening Checklist.
Adverse reactions to TMS are very unlikely (0.08% chance, or 8 in 10,000). Possible adverse reactions:
- Seizure – if this occurs it will usually begin during or immediately after the TMS test.
- Syncope – due to procedure-related anxiety.
- Mild headache – this is usually transient and resolves without analgesia.
These are adverse events of interest that ought to be documented using the VERIFY documentation.
Monitoring for adverse events
Monitor the participant for seizure and syncope throughout the TMS test. This can be done by visually observing the participant. Pay particular attention to any sudden changes in their level of alertness or any involuntary or unexpected movements of their limbs. Decreased alertness could be related to syncope, seizure, or simply falling asleep. Some people fall asleep during TMS testing. Gently rouse them with voice and/or light touch on their non-paretic upper limb as needed. Repeated involuntary or unexpected movements of their limbs could indicate a seizure. See the next section for planning your management of adverse events such as seizure.
When the TMS test is complete, note the time of the last stimulus, and continue observing the participant while you remove their EMG electrodes and pack up the TMS equipment.
Check on stroke patients 15 minutes and 30 minutes after the time when the TMS test was completed. TMS is considered complete after the last stimulation is delivered to the patient. These repeat checks involve observing their level of alertness and looking for any repeated involuntary or unexpected movements of their limbs.
Healthy volunteers during TMS training do not require clinical monitoring
Managing adverse events
Before you begin VERIFY you ought to have a plan in place for managing potential adverse events, no matter how unlikely they may be.
Seizure management will most likely involve calling the medical staff who will manage this event according to local protocol and procedures. Please establish an appropriate plan for your site in agreement with local medical staff.
You should also have a plan in place for managing syncope or a mild headache. The risks are minimal in both cases. The participant will be seated or in a bed and therefore well-supported if they experience syncope. If the participant volunteers that they are experiencing headache, reassure them that this is not unexpected and likely to be transient. They can be offered analgesia (such as acetaminophen) if their headache is troubling them and/or persistent. In both cases, communicate these events to the patient’s medical team for their information and prescription of analgesia if required.
If you require urgent assistance or advice during a TMS session please call or text the TMS hotline at: (833)337-2227 (Available Mon-Fri 8am-9pm ET)
